
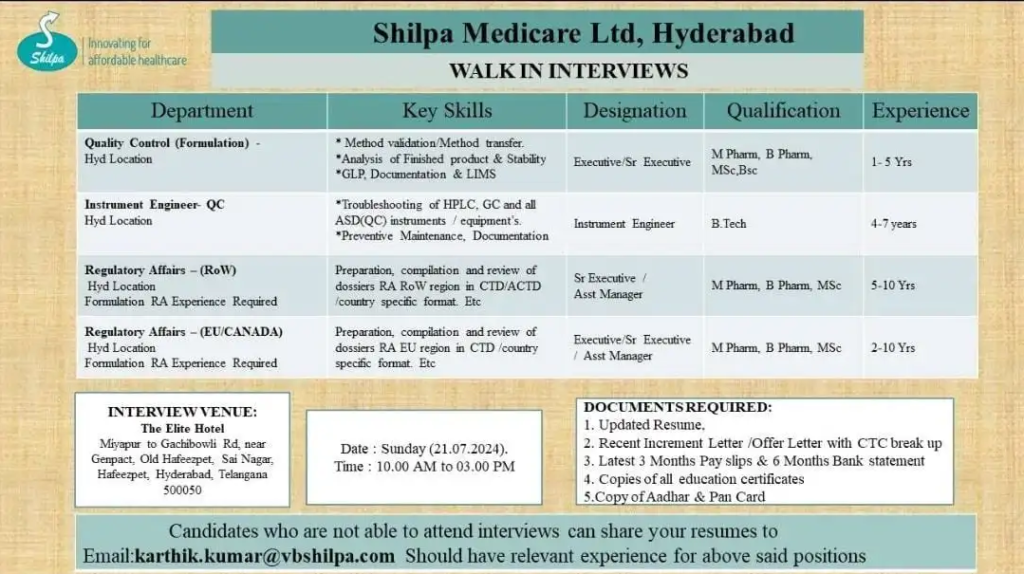
Shilpa Medicare walk in interview-Shilpa Medicare is excited to announce a walk-in interview for dynamic professionals in Quality Control (QC) and Regulatory Affairs for the EU, Canada, and Rest of the World (ROW) markets. This is an excellent opportunity to join a reputed pharmaceutical company and work on ensuring high standards of quality and regulatory compliance globally. If you have the expertise and are ready to take your career to the next level, come and be a part of Shilpa Medicare’s journey to excellence. Don’t miss out on this chance to grow with Shilpa Medicare!
-
Date:
- 27th July 2024
-
Time:
- 10.00 AM to 03.00 PM
-
Department:
- Quality Control (Formulation), Instrument Engineer QC, Regulatory Affairs – (ROW), Regulatory Affairs (EU/CANADA)
-
Education Qualification:
- Bsc/Msc,
- B.pharm/M.pharm,
- B.Tech.
-
Required Skills:
- Formulation RA Experience Required
-
Experience required:
- 1 to 10 Years
-
Benefits:
- NA
-
Documents required for interview:
- Kindly bring your resume,
- photocopy of education certificates,
- Copy of pan card
- Copy of Aadhar card,
- Copy of bank documents.
-
Venue:
-
Job Location:


